Silicate-Analogous Materials
In the focus of our research the investigation of structure-properties-relations of ionic solids of the groups 13–16 (e. g. borates, phosphates, sulphates and their derivatives) in the periodic table in combination with alkaline earth, transition and especially rare earth metals.
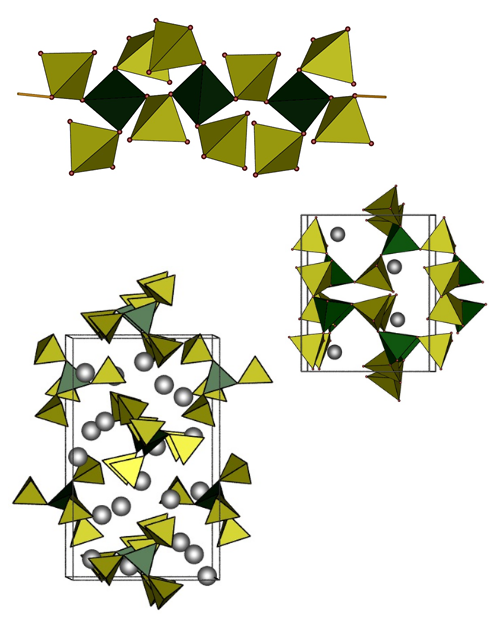
In silicate analogous compounds as basic structural motif tetrahedral building units are found; these do not comprise an inversion centre and lead thus preferentially to non-centrosymmetric surroundings of metal ions or non-centroysymmetric crystal structures in general. For efficient optical properties the absence of an inversion centre is helpful.
Target of our work are luminescent host compounds doped with rare-earth or transition metals (e. g. phosphors), solid acids for the heterogeneous catalysis as well as precursors for the mild and straight-forward synthesis of the previously mentioned materials. Moreover, we are interested in the structural chemistry and symmetry-relations of these new compounds.
The knowledge of the crystal structure of an investigated compound allows a certain synthesis design for the development of defined compounds with desired properties. A special focus lies therefore on the synthesis of phase-pure samples, the crystal growth of single-crystals and especially the structure analysis using X-ray diffraction.
Doped Host Materials as Phosphors
Actually the development of new phosphors concentrates on two main groups. On one hand rare-earth ions like Eu2+ are outstanding candidates for the synthesis of new classical fluorescent phosphors absorbing an UV photon and emitting up to one visible photon. On the other hand compounds of trivalent rare-earth ions (SE3+) may be suited for so-called quantum cutter materials which absorb a highly energetic UV photon and are capable of emitting more than one visible photon.
Based on these targets we are involved with the synthesis and characterisation of crystalline condensed borosulfates, fluorooxoborates, tungstates, sulfates, borates, borophosphates, phosphates and similar compounds of alkaline earth and rare-earth metals.
Classical Phosphors
We investigate phosphors based on divalent rare-earth ions. These exhibit 4f-5d transitions which are allowed by spin as well as parity selection rules and are therefore extraordinarily intense. Moreover, the participation of 5d transitions in the luminescent process enables to tune the emission wavelength via the chemical properties within the coordination sphere. Such compounds are promising phosphors for luminescent tubes or whith LEDs (light emitting diodes).
The luminescence of phosphors based on trivalent rare-earth ions are is due to 4f-4f transitions which are almost uninfluenced by the ligand field. But, occasionally the intensities of the interesting transitions are in close relation to the symmetry of the coordination environment.
Antenna Phosphors
Host structures comprising anions of heavy transition metals in high oxidation states are capable of being excited efficiently via charge transfer transitions. In case of suitably lying energy states of doped ios this energy may be transferred intensifying the luminescence of the activator ions. This mechanism is of specific interest for doped activator ions in which optical transitions are basically forbidden due to the parity or spin selection rules. Therefore, we are also interested in tungstates and relatives with regard of so-called antenna phosphors.
Quantum-Cutting-Phosphors
Due to environmental reasons Hg vapour lamps should be replaced by Xe high-pressure lamps. This requires the development of novel especially for the latter approach suited phosphors. The UV source emits at significantly higher energy (Xe high-pressure lamps, 147 nm) as compared with Hg vapour lamps (254 nm). A comparable energy efficiency can only be achieved if per absorbed UV photon more than one visible photon is emitted (quantum cutting). For such phosphors the band-gap has to be large enough and suited broad energy-levels with a high absorbance have to be present. Large coordination numbers of the metal ions additionally lead to moderate ligand field splittings of the present d levels.
Trivalent rare-earth ions have proven to be the activators of choice due to their weak interaction of the relevant 4f states with the host structure and the large number of accessible energy levels. Moreover SE3+ ions tend to larger coordination numbers. For certain reasons oxidic and flouridic host structures are the most promising ones.
Solid Acids
To the surface of solids bound protons may act as Brönsted acids. These are the stronger the more coordination sites are available for protons and the more electronegative the (poly-)anion of the solid state compound is. Analogously free coordination sites of electron deficient cations may act as strong Lewis acids enabling Lewis-Acid-Base adducts. Borates, phosphates and sulphates are predestined compounds for such solid state Bröndsted or Lewis acids and may be suited for the heterogeneous catalysis of chemical reactions.
Weakly Coordinating Host Structures
For many of above mentioned properties like solid acids, quantum cutting and antenna phosphors, sometimes also classical phosphors only weak or extremely weak interactions between host structure and activator (protons in case of acids) are desired. Accordingly, a focus of our current work is to investigate such weakly coordinating host structures like borosulfates and fluorooxoborates. The terminal atoms develop only weak electrostatic and covalent interactions.
Synthesis Methods
Our approaches for the synthesis of novel materials reach from classical solid state reactions in a variety of furnaces (up to approx. 2000 °C) via solvothermal syntheses to reactions in aqueous and non-aqueous solvents like Ionic Liquids. Furthermore we are interested in molecular precursor compounds of our materials which enable their synthesis under mild conditions or their defined deposition.
Structural Chemistry
We are inerested in concise structure descriptions of our solid state compounds using simple building units. Very advantageous are frequently symmetry relations with more or less chemically related but according to their symmetry related crystal structures. Using such relationships phase transitions may be described and classified.
Publications
A complete list of publications you also find here.